In the world of pharmaceutical manufacturing, few processes are as critical—and as misunderstood—as Commissioning, Qualification, and Validation (CQV). Whether you’re launching a new cleanroom, retrofitting a warehouse, or installing a critical utility system, CQV ensures that everything is “fit for its intended use” and compliant with GMP.
This article walks you through a modern, risk-based CQV process aligned with global standards such as EU GMP Annex 15, FDA Process Validation Guidance, ICH Q9, and ISPE Baseline® Guides.
What is CQV?
CQV stands for Commissioning, Qualification, and Validation, a structured process required in the pharmaceutical industry to ensure that facilities, equipment, utilities, and processes are fit for purpose, compliant with regulations, and able to consistently produce safe and effective medicines.
- Validation (V):
The final confirmation that processes, methods, and systems consistently achieve pre-defined outcomes. For instance, ensuring a drug manufacturing process produces tablets with the correct potency, purity, and stability every time.
- Commissioning (C):
The systematic process of verifying that equipment and systems are installed correctly, operate as intended, and meet design specifications. Example: Checking HVAC systems for proper airflow and sterile conditions.
- Qualification (Q):
The documented demonstration that equipment or systems meet defined quality standards. It usually includes:
IQ (Installation Qualification): Was it installed correctly?
OQ (Operational Qualification): Does it operate as specified?
PQ (Performance Qualification): Does it perform consistently in real conditions?
“CQV is not a one-time event. It’s an integrated lifecycle approach that ensures ongoing GMP compliance and product quality.”
— ISPE Baseline Guide, Vol. 5
1. Validation Master Plan (VMP): Your CQV Blueprint
A Validation Master Plan (VMP) is the blueprint for Commissioning, Qualification, and Validation (CQV) in the pharmaceutical industry, serving as a high-level document that defines the strategy, scope, responsibilities, and lifecycle of validation activities across facilities, equipment, utilities, and processes. It ensures regulatory compliance (FDA, EMA, WHO), provides clarity for cross-functional teams, and organizes validation efforts through risk-based approaches (ICH Q9, ASTM E2500). By outlining what needs validation, how it will be executed (IQ, OQ, PQ, ongoing verification), and who is responsible, the VMP centralizes documentation, avoids inconsistencies, and reassures regulators that the company has a structured and compliant quality system—making it the essential CQV roadmap.
This foundational document outlines:
- Project scope and boundaries
- Validation strategy (risk-based or full lifecycle)
- Roles and responsibilities
- Acceptance criteria
- Document hierarchy
ICH Q9 recommends a risk-based approach—prioritizing systems that directly impact product quality and patient safety.
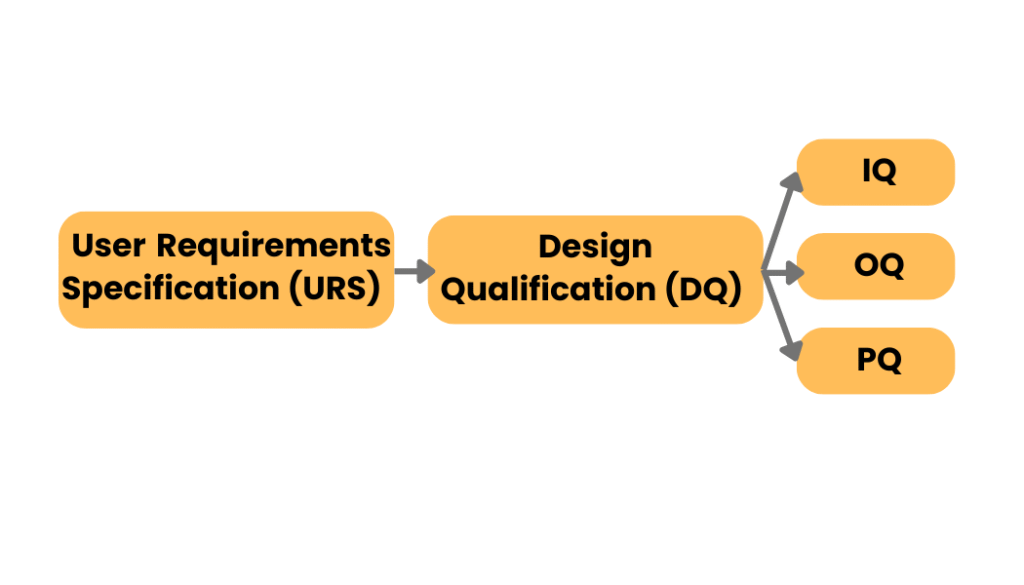
2. URS and DQ: Designing for Compliance
A User Requirement Specification (URS) is the documented voice of the user. It defines what a system, equipment, or facility must do to support the intended process. Once the URS is approved, a Design Qualification (DQ) demonstrates and documents that the proposed design will meet those requirements—under both normal and worst-case conditions.
In pharmaceutical projects, URS and DQ are essential steps in designing for regulatory compliance.
- The URS clearly states what the end user needs, covering aspects such as performance, safety, capacity, data integrity, and regulatory expectations.
- The DQ then verifies that the proposed design—whether for equipment, cleanrooms, utilities, or computerized systems—meets the URS and complies with GMP regulations.
Together, URS and DQ ensure that compliance and quality are embedded from the outset, reducing the risk of costly modifications later and providing documented evidence to regulators that systems were designed with product quality and patient safety in mind.
Example – Cleanroom Project:
A pharmaceutical company is building a cleanroom for sterile injectable production. In the URS, the company specifies: ISO Class 5 air cleanliness in critical zones, strict control of temperature and humidity, pressure differentials to prevent contamination, and compliance with FDA and EU GMP. During the DQ, engineers and QA review HVAC design, material and personnel flows, cleanroom layouts, and monitoring systems. They confirm that air handling units can maintain HEPA filtration performance, pressurization prevents cross-contamination, and sensors are appropriately located for continuous monitoring. By documenting this alignment, the company proves that the design meets user and regulatory requirements before construction begins.
Role of QA: Quality Assurance ensures that no equipment, facility, or software is purchased or built until the DQ has been formally approved.
3. Commissioning: Make It Work Before You Validate It
Commissioning in pharmaceutical projects is the structured process of making sure that facilities, utilities, systems, or equipment are installed and operate correctly before formal validation begins. It involves checking that everything works as intended—verifying installation, testing functions, and ensuring performance under expected operating conditions. In other words, commissioning is about “making it work” by identifying and fixing issues early, so that by the time validation starts, the system is already reliable, compliant, and ready to demonstrate consistently that it meets regulatory and user requirements. This prevents delays, reduces costs, and ensures a smoother validation phase.
Checklist includes:
- Installed as per drawing
- Sensor and probe calibration
- HVAC and alarm system testing
- Closing punch list items
- Pre-OQ functional testing
here’s a real-world commissioning example for an HVAC system in pharma:
A pharmaceutical company is constructing a cleanroom for sterile production. During HVAC commissioning, engineers verify that air handling units (AHUs) are installed correctly, ductwork is sealed, and HEPA filters are properly fitted. They test airflow volumes, pressure differentials between rooms, and confirm that temperature and humidity can be controlled within specified limits. Smoke tests are performed to visualize airflows and ensure there are no turbulence zones or backflows that could risk contamination. Any deviations, such as insufficient pressure differentials or poor filter sealing, are corrected at this stage. By completing commissioning first, the HVAC system is stable and reliable, allowing validation to then formally prove compliance with GMP requirements (ISO cleanroom classification, FDA/EU Annex 1, etc.).
4. IQ → OQ → PQ: The Core of Qualification
IQ → OQ → PQ represent the three core stages of qualification in pharmaceutical validation.
- Installation Qualification (IQ) confirms that equipment or systems are installed correctly according to design specifications, manufacturer recommendations, and GMP requirements.
- Operational Qualification (OQ) then verifies that the system operates as intended across all specified operating ranges, including alarms, controls, and worst-case conditions.
- Performance Qualification (PQ) demonstrates that the system, when integrated into actual production, consistently performs according to user requirements and regulatory standards.
Together, IQ, OQ, and PQ provide documented evidence that facilities, equipment, and systems are fit for their intended use, ensuring product quality and patient safety. These steps must be fully documented with traceability to the URS.
5. Don’t Forget the Software & Utilities
In modern pharmaceutical facilities, validation is not limited to production equipment alone—software systems and utilities are equally critical and must be qualified to ensure compliance and product quality. Core digital platforms such as the Environmental Monitoring System (EMS), which tracks cleanroom parameters like temperature, humidity, and particle counts, the Building Management System (BMS), which controls HVAC and facility conditions, the Warehouse Management System (WMS) for material handling and traceability, and Computerized Maintenance Management Systems (CMMS), which schedule and document preventive maintenance, all fall under computerized system validation.
These must be validated according to GAMP 5 principles, ensuring systems are reliable, fit for intended use, and compliant with data integrity standards.
Likewise, critical utilities—such as clean steam, compressed air, water for injection (WFI), and nitrogen—must undergo full qualification (IQ, OQ, PQ) to prove they consistently meet defined purity and performance specifications. Additionally, electronic systems must comply with 21 CFR Part 11 (FDA) and EU Annex 11, which regulate electronic records and signatures, ensuring data traceability, security, and regulatory acceptability.
By integrating validation of both digital systems and utilities, companies ensure that all supporting infrastructure is robust, compliant, and capable of protecting product quality and patient safety.
6. Maintaining the Validated State
In pharmaceutical manufacturing, Maintaining the Validated State within the Commissioning, Qualification, and Validation (CQV) lifecycle ensures that facilities, utilities, equipment, processes, and computerized systems consistently perform as intended and remain compliant with regulatory requirements throughout their operational life.
After initial validation, the validated state must be sustained through a robust Continued Process Verification (CPV) program, including periodic review of critical process parameters, preventive maintenance, calibration, change control, and deviation management. Any changes in equipment, materials, or processes must be evaluated for impact on validation, triggering requalification or revalidation when necessary.
Regulatory agencies (FDA, EMA, ICH Q7, Q9, Q10) emphasize that validation is not a one-time event but an ongoing effort, ensuring product quality, patient safety, and data integrity across the product lifecycle.
Validation isn’t a checkbox—it’s a living state. Maintain it with:
- SOPs for calibration, deviation, and corrective action
- Preventive maintenance via CMMS
- Periodic requalification (e.g. annual mapping of cold storage)
- Risk-based change control for facility updates
“Changes must be controlled to ensure they don’t adversely impact product quality or validated conditions.”
— EU GMP Annex 15, Clause 13
7. QA Release & Final Documentation
QA Release & Final Documentation in pharmaceutical manufacturing is the culmination of the CQV process, ensuring that all systems, equipment, and processes are fully qualified, validated, and compliant before being used in GMP production. At this stage, the Quality Assurance (QA) team performs a comprehensive review of all documentation, including protocols, reports, deviations, and change controls, to verify accuracy, completeness, and regulatory compliance. The final success of CQV depends on several critical deliverables:
- Training records for all impacted staff (Operations, QA, IT, Engineering) to confirm readiness.
- A Validation Summary Report (VSR) consolidating all qualification and validation activities with conclusions.
- A traceability matrix mapping each executed test back to its corresponding User Requirement Specification (URS) item.
- Closed deviations and change requests, ensuring no unresolved issues remain.
- Formal QA approval declaring the system “fit for intended GMP use.”
Only after these elements are verified and documented does QA issue the release decision, authorizing GMP use and providing the regulatory assurance of product quality, safety, and compliance.
Conclusion: Validate Smart. Validate Once.
Whether you’re scaling a biotech suite or launching a new fill-finish line, CQV remains your first line of defense in GMP compliance. By integrating engineering discipline, quality oversight, and risk management principles, CQV not only ensures that systems are robust and reliable but also helps organizations shorten audits, maintain regulatory trust, and safeguard patients. The key is to start with a clear validation master plan, align it with international standards (ICH Q8–Q10, ISPE Baseline Guides, FDA/EMA guidance), and execute with rigor. Done correctly, CQV is not just a regulatory requirement—it is a strategic advantage, enabling you to validate smart, validate once, and build sustainable GMP operations.