End-to-End Services: Modular Cleanroom Facilities
Design & Engineering
Faster, Smarter, and More Sustainable Construction
Modular construction transforms the way pharmaceutical and high-tech facilities are built—by shifting most of the work off-site to a controlled factory environment. This innovative approach significantly reduces on-site disruption while enhancing speed, compliance, quality, and efficiency.
- Accelerated Timelines: Projects are completed 30–50% faster than traditional methods
- Cost Savings: Reduce total building costs by up to 20%
- Labor Efficiency: Cut on-site labor needs by up to 45%
Facilities are designed to meet USFDA, EU GMP, cGMP, ISPE, WHO and guidelines.
Process Understanding Design
Translating user requirements into cGMP-compliant facility designs.
Conceptual to Detailed Design
Developing Process Flow Diagrams (PFDs), Piping and Instrumentation Diagrams (P&IDs), and 3D Building Information Modeling (BIM).
Engineering Excellence
Multi-Disciplinary Expertise: Teams with extensive experience design principles aligned with AIA (American Institute of Architects) framework.
Integrated Modular Cleanroom Facility Components
Modules can be configured individually or clustered to meet specific production needs.
Each modular unit includes fully equipped production and technical areas with a specific design, featuring:
Off-Site Modular Construction
Fully Integrated Modular Pharmaceutical Facility
Our pharmaceutical facilities are expertly divided into self-contained modules, each built with a pre-finished steel structure, fabricated roofing, staircases, and an external façade clad in aluminum composite panels for durability and aesthetic appeal.
Off-Site Construction in 5 Expert Stages:
The pre-finished modular units are structurally engineered to withstand the demands of any environment—supporting all utilities and equipment while accommodating seismic, wind, and snow loads specific to the facility’s destination.
Every facility is meticulously designed to meet cGMP manufacturing standards and complies with key international guidelines, including: EU GMP, WHO, ISPE, ASHRAE. This ensures full regulatory alignment and operational excellence from day one.
Structural Fabrication
⇒
MEP Integration (Mechanical, Electrical & Plumbing) ⇒
Cleanroom Fit-Out & Equipment Installation ⇒
Testing & Validation (Including FAT) ⇒
Shipping & On-Site Assembly
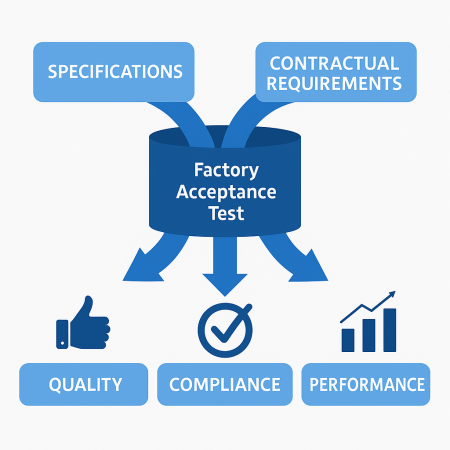
Validation & Factory Acceptance Testing (FAT)
Quality Assured Before Delivery
In our modular facilities, Factory Acceptance Testing (FAT) and Validation are performed off-site before shipment—ensuring faster deployment and regulatory compliance.
FAT focuses on testing equipment functionality and performance against design specifications.
Validation ensures that processes consistently produce products meeting quality and cGMP standards.
This proactive approach reduces installation errors, shortens project timelines, and lowers the risk of regulatory non-compliance—delivering a fully operational, quality-assured facility from day one.
On-Site Installation
The Modular Facility is designed for fast, efficient on-site installation—ensuring a strong start and an epic finish. The process includes four key phases:
Unloading, Base Preparation, Placement, and Inter-Module Connections & Commissioning.
Each module is pre-engineered for easy transport and assembly, minimizing disruption and preserving process flow. A reinforced concrete foundation and structural supports ensure long-term stability, making the installation swift, secure, and scalable.

Project Management
We offer comprehensive project management led by a single dedicated point of contact, ensuring clear communication and accountability. Our team combines technical excellence in design, engineering, and compliance with effective planning and execution.
From initial concept to final installation, we handle:
Design coordination
Engineering and procurement
Scheduling and cost control
On-site supervision and commissioning
This streamlined approach guarantees faster timelines, regulatory compliance, and cost-effective results—delivering your modular facility efficiently and reliably.
Pharmaceutical Procurement & Supply Chain Solutions
We manage end-to-end procurement for pharmaceutical manufacturing, sourcing equipment, machinery, APIs, and critical materials. Through strategic partnerships and industry expertise, we deliver cost-effective, compliant, and timely supply, ensuring smooth operations and faster time-to-market.
Pharmaceutical Tech Transfer
We support biotech innovators and global pharma companies with end-to-end technology and API transfer services—enabling smooth, compliant scale-up and rapid entry into new markets. Whether you’re moving from lab to GMP manufacturing or expanding production across borders, we streamline the process to accelerate time-to-market and ensure regulatory success.
Customers services
From project execution to full operation, we’re with you every step of the way.
Our support services begin during the project and continue long after handover. With fast response times, proactive maintenance, and expert assistance, we help you minimize downtime and maintain peak productivity.
With a single dedicated partner, you gain reliable support, seamless coordination, and long-term performance, from day one and well into the future
We deliver on demand tailored training programs covering GMP compliance, QA, QC, equipment operation, and maintenance best practices, ensuring your team is confident and your facility runs smoothly.
CONTACT US
Whether you’re looking to expand your pharmaceutical manufacturing capacity, design modular cleanroom solutions, or procure specialized equipment and APIs, we’re here to help!
Reach Us
Location :
Houston, Texas, USA
Email :
info@biotheralifesciences.com
Phone :
+1-281-300-2459
